The microbial electrosynthesis cell that turns carbon dioxide gas into valuable chemicals was created by (L-R) Krishna Katuri, Pascal Saikaly, Bin Bian and Manal Alqahtani.
The microbial electrosynthesis cell that turns carbon dioxide gas into valuable chemicals was created by (L-R) Krishna Katuri, Pascal Saikaly, Bin Bian and Manal Alqahtani.
Carbon emissions smoked
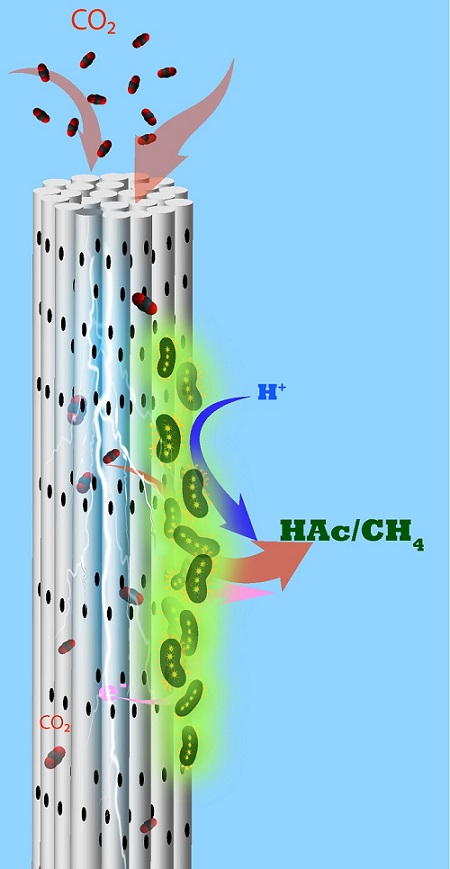
Helpful microbes inhale CO2 through a porous cylindrical electrode and exude useful chemicals.
Microbes could become key allies in global efforts to curb carbon emissions and avoid dangerous climate change. A group of microbes called chemolithoautotrophs consumes CO2 through their natural metabolism, spitting out small organic molecules as a byproduct. These microbes could be enlisted to convert industrial CO2 emissions into valuable chemicals, thanks to a new concept developed by Pascal Saikaly and his team at KAUST.
Chemolithoautotrophs are commonly found in the deep sea, in caves and hydrothermal vents, where conventional energy sources, such as sunlight and organic carbon, are lacking. “The microbes obtain their energy from the oxidation of inorganic compounds, such as hydrogen, iron and sulfur,” explains Bin Bian, a Ph.D. student from Saikaly’s team. The microbes strip the inorganic compounds of electrons while taking up CO2 and reducing it to organic products as part of the process.
Microbes growing on porous cylindrical electrodes suck in CO2 and turn it into
useful chemicals such as acetate and methane.
To harness chemolithoautotroph capabilities for recycling CO2 emissions into useful chemicals, researchers supply electrons to the microbes in a process called microbial electrosynthesis (MES). Typically, MES reactors have grown chemolithoautotrophs on a submerged flat-sheet cathode and bubbled CO2 gas into the solution, but this setup has two key limitations, explains Manal Alqahtani, also a Ph.D. student in the team. Flat-sheet cathodes are difficult to scale up and CO2 gas has poor solubility.
The team developed an alternative MES reactor using cathodes made from stackable, cylindrical porous nickel fibers that Saikaly’s group had previously applied to recover water and energy from wastewater1. CO2 is pumped through each cylinder, and electrons flow along it. “Using this architecture, we directly deliver CO2 gas to chemolithoautotrophs through the pores in the hollow fibers,” Alqahtani says. “We provided electrons and CO2 simultaneously to chemolithoautotrophs on the cathode surface.”
In Alqahtani’s initial study, methane-producing microbes were able to convert CO2 to methane with 77 percent efficiency, compared to 3 percent efficiency with a conventional design.
A follow-up study improved performance further by coating the electrodes with carbon nanotubes2. These offered a more biocompatible surface for microbial growth and improved the hollow fibers’ CO2 adsorption capability 11-fold. “Additionally, the nanotubes enhanced the electron transfer from electrode to chemolithoautotrophs,” Bin says. In tests using acetate-producing microbes, production of the chemical almost doubled when the nanotube coating was applied, he says.
Alqahtani’s ongoing work includes investigating easier approaches to develop porous cylindrical cathodes, while Bian is optimizing CO2 flow rates and investing renewable MES energy sources, such as solar. Both students acknowledge the valuable contribution made to their studies by Krishna Katuri, a research scientist in Saikaly’s lab.




 The microbial electrosynthesis cell that turns carbon dioxide gas into valuable chemicals was created by (L-R) Krishna Katuri, Pascal Saikaly, Bin Bian and Manal Alqahtani.
The microbial electrosynthesis cell that turns carbon dioxide gas into valuable chemicals was created by (L-R) Krishna Katuri, Pascal Saikaly, Bin Bian and Manal Alqahtani.